mikey
Member
Multiple chemical agents are involved in caustic alkali burns. Lime, potassium hydroxide, and sodium hydroxide are the most common agents causing chemical injury. Accidental injury occurs in exhaust Hood Cleaners. Among chemical burns, alkali injuries occur frequently and are likely to cause severe symptoms. The mechanism by which these alkali injuries are caused is due to three factors:
saponification of fat causes fatty tissue to lose its function with increased damage due to heat reaction.
extraction of water from cells causing dessication
bind with the proteins of the tissues to form alkaline proteinates.
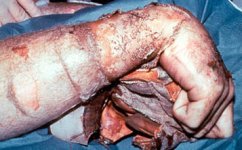
The extent of the damage caused by an alkali substance depends on its concentration, amount, and time of contact with the skin.
As with other chemical burns, alkalis are capable of deep penetration, and can cause severe pain. For treatment, it is necessary to remove the causative substance as quickly as possible by washing with large volumes of water. Washing is presumed to cause dilution and elimination of a chemical substance.
The initial treatment for burns caused by strong alkaline solution is copious irrigation with water. Water may dissipate any heat by dilution so as to prevent further damage. In lime burns, the dry lime must be brushed away before washing in order to minimize the production of heat. The most readily available material for removing a chemical agent is water, and it is extremely important to remove a chemical agent as
rapidly as possible. Washing with large quantities of water is the preferred treatment. It is very difficult to determine the depth of tissue damage on admission. The corrosive material blackens the skin, rapidly converting it to a hard, dry eschar.
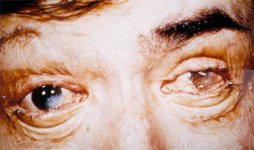
Ocular damage is a common sequel to alkali injury. Damage is related to the pH: the higher the pH, the greater the damage to the eye. The corrosive agent trickles down the eyelid and enters the acanthi. Alkalis penetrates quickly and, with little resistance.
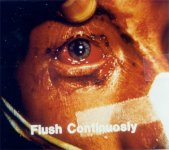
Such damage may be made worse by repeated attempts to wipe off the chemical agent with the hands or inadequately washing the eyes. The policy is to treat with topical anesthetics followed by copious irrigation, inducation of cycloplegia and mydriasis as soon as possible.
saponification of fat causes fatty tissue to lose its function with increased damage due to heat reaction.
extraction of water from cells causing dessication
bind with the proteins of the tissues to form alkaline proteinates.
The extent of the damage caused by an alkali substance depends on its concentration, amount, and time of contact with the skin.
As with other chemical burns, alkalis are capable of deep penetration, and can cause severe pain. For treatment, it is necessary to remove the causative substance as quickly as possible by washing with large volumes of water. Washing is presumed to cause dilution and elimination of a chemical substance.
The initial treatment for burns caused by strong alkaline solution is copious irrigation with water. Water may dissipate any heat by dilution so as to prevent further damage. In lime burns, the dry lime must be brushed away before washing in order to minimize the production of heat. The most readily available material for removing a chemical agent is water, and it is extremely important to remove a chemical agent as
rapidly as possible. Washing with large quantities of water is the preferred treatment. It is very difficult to determine the depth of tissue damage on admission. The corrosive material blackens the skin, rapidly converting it to a hard, dry eschar.
Ocular damage is a common sequel to alkali injury. Damage is related to the pH: the higher the pH, the greater the damage to the eye. The corrosive agent trickles down the eyelid and enters the acanthi. Alkalis penetrates quickly and, with little resistance.
Such damage may be made worse by repeated attempts to wipe off the chemical agent with the hands or inadequately washing the eyes. The policy is to treat with topical anesthetics followed by copious irrigation, inducation of cycloplegia and mydriasis as soon as possible.